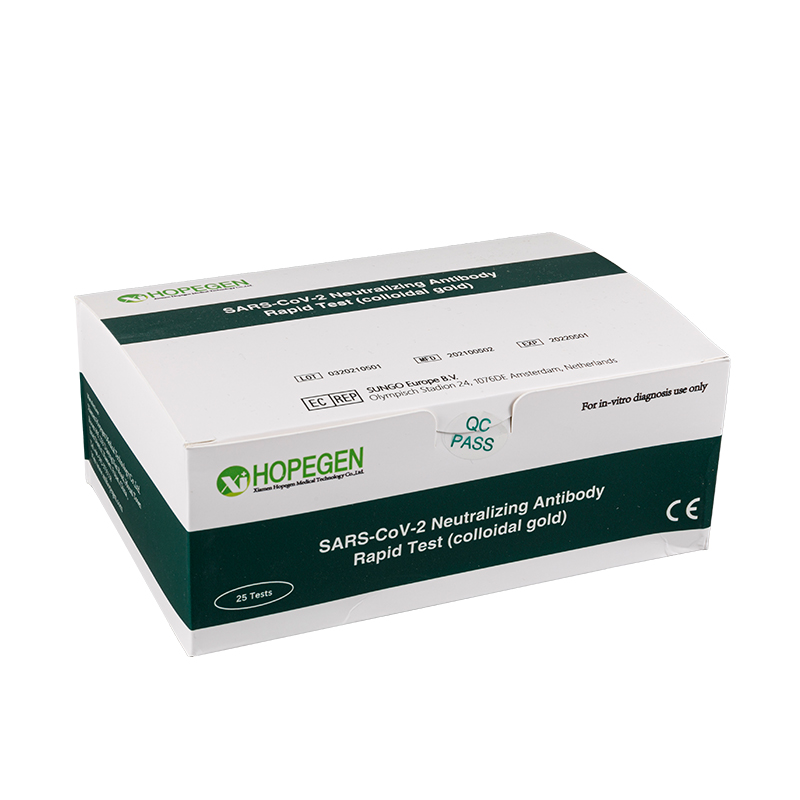
CE Certification Surgical Disposable Factories – COVID-19 Antigen Rapid Test Kit – CILIANG
CE Certification Surgical Disposable Factories – COVID-19 Antigen Rapid Test Kit – CILIANG Detail:
Intended use
COVID-19 Antigen Rapid Test Kit (Colloidal Gold) is used for in vitro qualitative detection of the SARS-CoV-2 antigen (Nucleocapsid protein) in human nasal swabs/ oropharyngeal swabs specimen.
The novel corona virus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Test principle
This kit uses immunochromatography for detection. The specimen will move forward along the test card under capillary action. If the specimen contains SARS-CoV-2 antigen, the antigen will bind to the colloidal gold-labeled new corona virus monoclonal antibody. The immune complex will be captured by corona virus monoclonal antibodies which are membrane fixed, form the fuchsia line in detection line, display will be SARS-CoV-2 antigen positive; if the line does not show color, and it means the negative result. The test card also contains a quality control line C, which shall appear fuchsia regardless of whether there is a detection line.
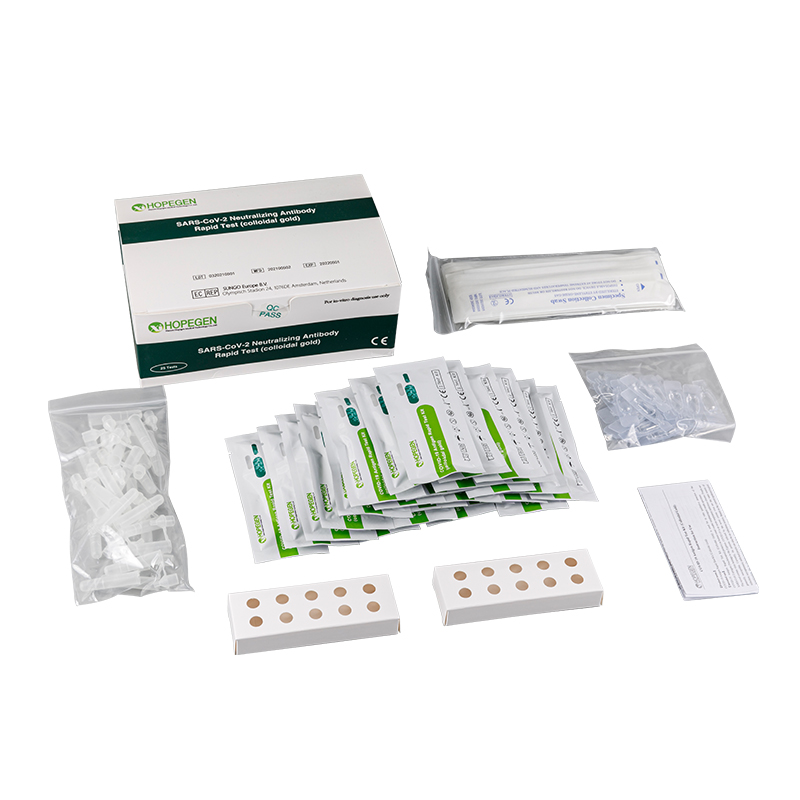
Specifications and Main Components
| SpecificationComponent | 1 Test/Kit | 5 Tests/Kit | 25 Tests/Kit |
| COVID-19 Antigen Test Card | 1 piece | 5 pieces | 25 pieces |
| Extraction Tube | 1 piece | 5 pieces | 25 pieces |
| Extraction R1 | 1 bottle | 5 bottles | 25 bottles |
| Instructions for Use | 1 copy | 1 copy | 1 copy |
| Disposable Swab | 1 piece | 5 pieces | 25 pieces |
| Tube Holder | 1 unit | 2 units |
Storage and Validity Period
1.Store at 2℃~30℃, and it is valid for 18 months.
2.After the aluminum foil bag is unsealed, the test card should be used as soon as possible within one hour.
Test Methods
The test method was colloidal gold. Please read the manual and the instrument operation manual carefully before use.
1.Open the package and take out the test card.
2.Place the extraction tube into the Tube Holder of the carton.
3.Rotate the lid of the swab extractor bottle(R1).
4.Squeeze all extraction solution out of the bottle into the extraction tube.
5.Put the swab specimen into the extraction tube, rotate the swab for about 10 seconds, and press the swab head against the tube wall to release the antigen in the swab. Squeeze the swab over the head to remove the swab so as to remove as much liquid as possible from the swab. Dispose of swabs according to biohazard waste disposal method.
6.Install the beater on the extraction tube, put two drops into the specimen hole of the test card, and start the timer.
7.Read the results within 20 minutes. Strong positive results can be reported within 20 minutes, however, negative results must be reported after 20 minutes, and the results after 30 minutes are no longer valid.

Product detail pictures:
Related Product Guide:
Our personnel are always in the spirit of "continuous improvement and excellence", and together with the top-quality good quality solutions, favorable selling price and superior after-sales providers, we try to acquire each customer's rely on for CE Certification Surgical Disposable Factories – COVID-19 Antigen Rapid Test Kit – CILIANG, The product will supply to all over the world, such as: Lebanon, Israel, Costa Rica, With a wide range, good quality, reasonable prices and stylish designs, our products are extensively used in this field and other industries. We welcome new and old customers from all walks of life to contact us for future business relationships and achieving mutual success! We welcome customers, business associations and friends from all parts of the world to contact us and seek cooperation for mutual benefits.
This is a honest and trustworthy company, technology and equipment are very advanced and the prodduct is very adequate, there is no worry in the suppliment.



