OEM PCR Equipment Factories – COVID-19 Antigen Rapid Test Kit – CILIANG
OEM PCR Equipment Factories – COVID-19 Antigen Rapid Test Kit – CILIANG Detail:
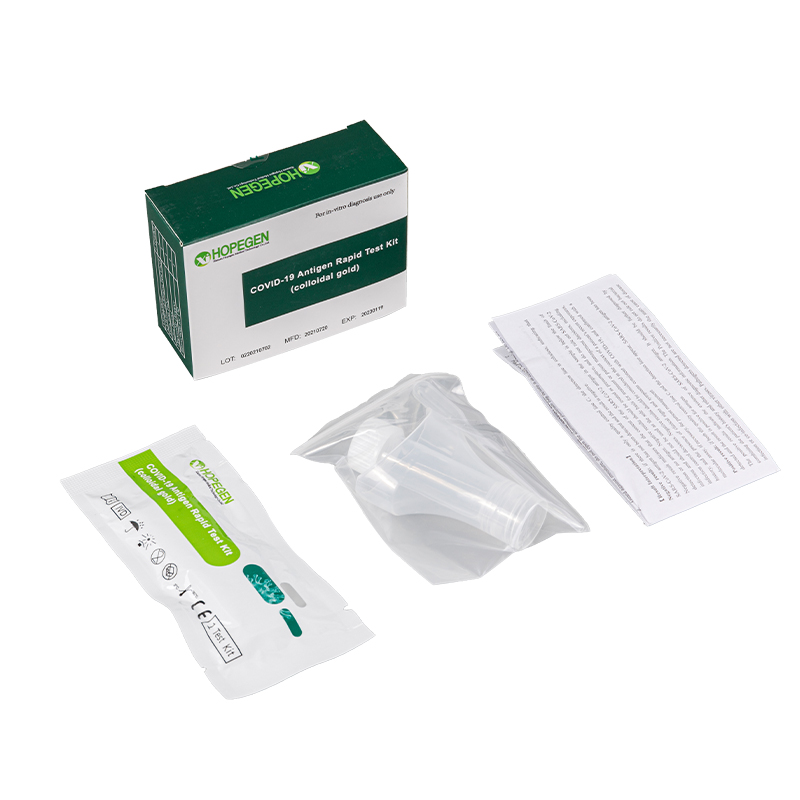
(Colloidal Gold)-1test/kit [saliva collection]

Test Methods
The test method was colloidal gold. Please read the manual and the instrument operation manual carefully before use.
1.Open the package and take out the test card.
2.Place the extraction tube(include collected saliva) into the Tube Holder of the carton.
3.Open the lid and draw a tube of liquid with a disposable dropper. Drop 2 drops into the sample well of the test card and start the timer.
4.Read the results within 20 minutes. Strong positive results can be reported within 20 minutes, however, negative results must be reported after 20 minutes, and the results after 30 minutes are no longer valid.

Result Interpretation
Negative result: if there is only a quality control line C, the detection line is colorless, indicating that SARS-CoV-2 antigen has not been detected and the result is negative.
Negative result indicates that the content of the SARS-CoV-2 antigen in the sample is below the limit of detection or no antigen. Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
Positive result: if both the quality control line C and the detection line appear, SARS-CoV-2 antigen has been detected and the result is positive for antigen.
The positive results indicate the existence of SARS-CoV-2 antigen. It should be further diagnosed by combining the patient’s history and other diagnostic information. The Positive results do not rule out bacterial infection or co-infection with other viruses. Pathogens detected are not necessarily the main cause of disease symptoms.
Invalid result: if the quality control line C is not observed, it will be invalid regardless of whether there is detection line (as shown in the figure below), and the test shall be conducted again.
The invalid result indicates that the procedure is not correct or that the test kit is out of date or invalid. In this case, the package insert should be read carefully and repeat.

The test with a new test device. If the problem persists, discontinue using the test kit of this Lot number immediately and contact your local distributor.
Product detail pictures:

Related Product Guide:
We have the most advanced production equipment, experienced and qualified engineers and workers, recognized quality control systems and a friendly professional sales team pre/after-sales support for OEM PCR Equipment Factories – COVID-19 Antigen Rapid Test Kit – CILIANG, The product will supply to all over the world, such as: Manchester, United Arab Emirates, Brunei, With excellent products, high quality service and sincere attitude of service, we ensure customer satisfaction and help customers create value for mutual benefit and create a win-win situation. Welcome customers all over the world to contact us or visit our company. We will satisfy you with our professional service!
Sales manager is very enthusiastic and professional, gave us a great concessions and product quality is very good,thank you very much!